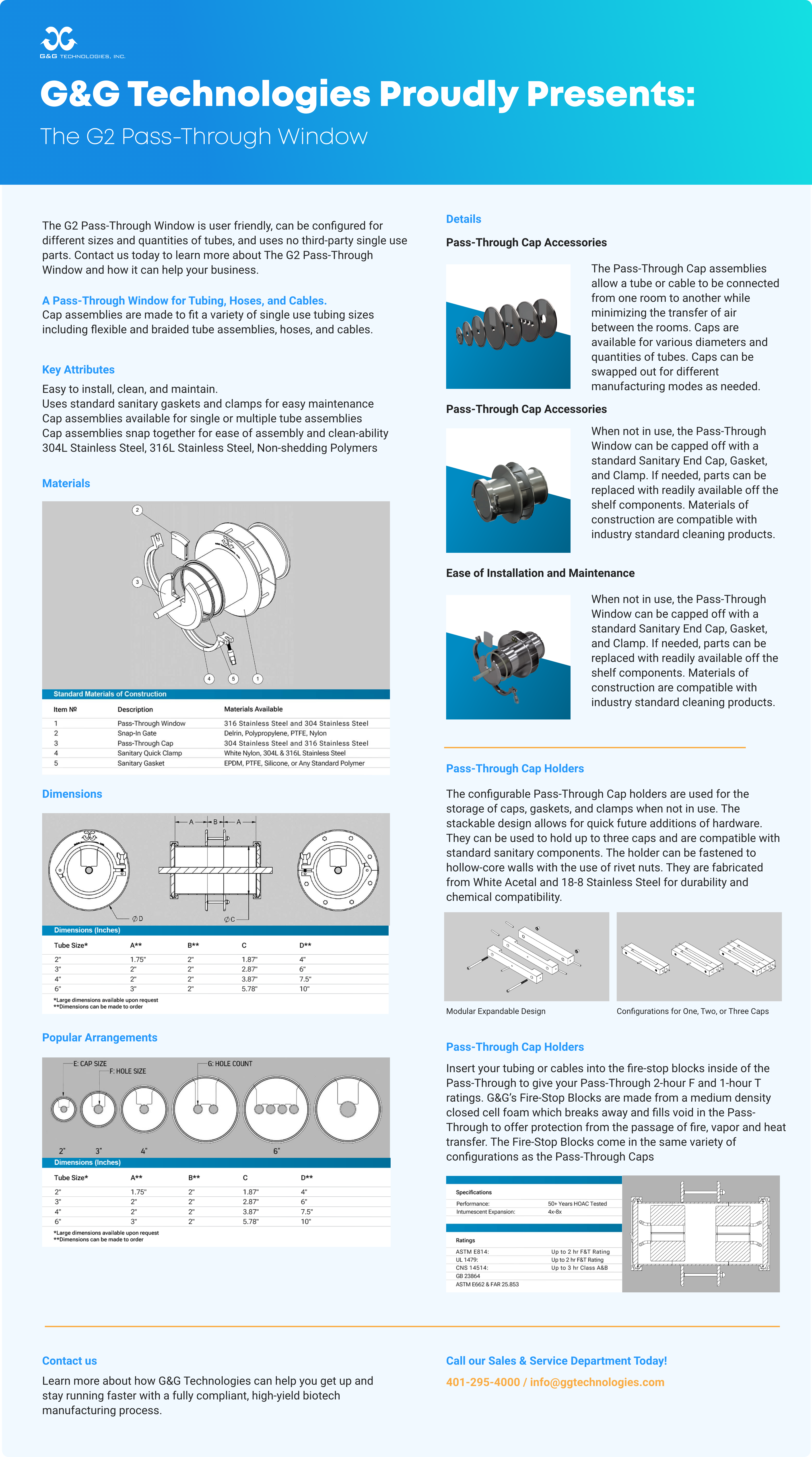
Today we develop
for a better future
We are grounded in both the disciplines of meticulous systems design and cGMP, we can better help our customers achieve their most important goals of bioprocessing system process optimization and regulatory compliance.
We can help translate your objectives into a viable process design concept and connect you with resources for implementing it. Once it’s up and running, we can provide resources to optimize process efficiency and minimize safety risks.
G&G Service Specialists are your insurance policy against unplanned downtime and excessive Operating & Maintenance costs.
Our Products
G&G Technologies has the capability of supplying a wide range
of process equipment solutions and services.
Single-Use Systems
Fluid Handling Solutions
Conventional Process Systems
Made-To-Order Items
New Developments
Contact US


Follow us on LinkedIn